Chemistry Journal of Moldova
SYNTHETIC ZEOLITES MODIFIED WITH SALTS OF TRANSITION METALS IN THE REACTION OF CHEMISORPTION-CATALYTIC OXIDATION OF SULPHUR DIOXIDE BY AIR OXYGEN
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2021 Volume 16, no.2
Pages: 91-101
Tatyana Rakitskaya, Tatyana Kiose, Lyudmila Raskola
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2021 Volume 16, no.2
Pages: 91-101
sulphur dioxide, synthetic zeolite, oxidation, chemisorption-catalytic process, transition metal.
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: The effect of the nature and concentration of d-metal salts attached to synthetic zeolites NaA and KA on the kinetic and stoichiometric parameters of the chemisorption-catalytic oxidation of sulphur dioxide with air oxygen at ambient temperature was studied. It was found that the adsorption capacity of NaA zeolite relative to SO2 is 100 times higher than that of KA zeolite; the time of protective action of NaA and KA zeolites increases upon modification with transition metal salts and with an increase of their content in the compositions. It was shown that the formation of inner and outer sphere complexes and the relationship between them is determined by the nature and concentration of metal ions and by the nature of the carrier. It was proven that the chemisorption-catalytic process ends with the oxidation of SO2 to H2SO4.
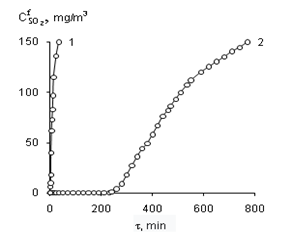
Graphical Abstract: The effect of the nature and concentration of d-metal salts attached to synthetic zeolites NaA and KA on the kinetic and stoichiometric parameters of the chemisorption-catalytic oxidation of sulphur dioxide with air oxygen at ambient temperature was studied. It was found that the adsorption capacity of NaA zeolite relative to SO2 is 100 times higher than that of KA zeolite; the time of protective action of NaA and KA zeolites increases upon modification with transition metal salts and with an increase of their content in the compositions. It was shown that the formation of inner and outer sphere complexes and the relationship between them is determined by the nature and concentration of metal ions and by the nature of the carrier. It was proven that the chemisorption-catalytic process ends with the oxidation of SO2 to H2SO4.
Downloads: 90






