Chemistry Journal of Moldova
ANTIOXIDANT PROPERTIES OF DIHYDROXYFUMARIC ACID AND ITS DIMETHYL ETHER: A COMPARATIVE DFT STUDY OF THEIR REACTIONS WITH THE STABLE RADICAL DPPH*
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2015 Volume 10, no.1
Pages: 89-94
Mikhail Gorbachev, Natalia Gorinchoy, Ion Arsene
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2015 Volume 10, no.1
Pages: 89-94
antioxidant activity, DPPH*, dihydroxyfumaric acid, dimethyl ether of dihydroxyfumaric acid, DFT calculations.
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2015.10(1).13
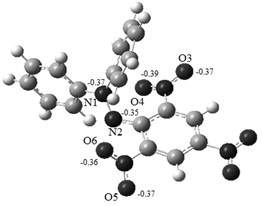
Graphical Abstract: The preferred mechanism of the reaction of dihydroxyfumaric acid and its dimethyl ether with the free stable radical 1,1-diphenyl-2-picrylhydrazyl (DPPH*) was revealed by means of Density Functional Theory (DFT) calculations. The proposed mechanism has an ionic character and includes the formation of charge-transfer complexes as the main stage. It is also shown that the lower antioxidant activity of dimethyl ether of dihydroxyfumaric acid is caused by both its lower acidity (as compared with its precursor acid) and formation of more stable intermediates during its reaction with DPPH*. Our results allow one to rationalize the available experimental data.
Downloads: 73






