Chemistry Journal of Moldova
DFT STUDY OF THE ENTIRE REACTION CYCLE OF H2O2 DECOMPOSITION AND O2 GENERATION CATALYZED BY FENTON REAGENT
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 88-97
Ion Arsene, Natalia Gorinchioy
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 88-97
Fenton reaction, H2O2 decomposition, DFT calculations
Full Text (PDF): Download
Abstract (PDF)
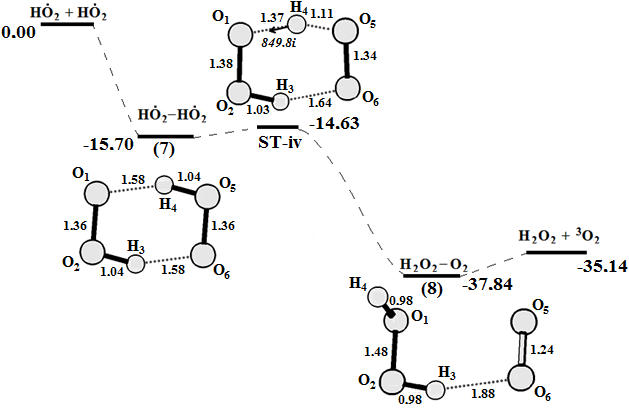
Graphical Abstract: The reaction cycle of H2O2 decomposition and O2 generation catalyzed by Fenton reagent was studied using density functional theory calculations. A four-stage mechanism for the oxygen production and the Fe2+ regeneration in the Fenton reaction is proposed based on the obtained results. It is shown that the O-O bond cleavage of coordinated H2O2at the first step of reaction does not lead to a free HO● radical. Instead, a highly reactive intermediate [FeIV(H2O)4(OH)2]2+ with two HO● radicals “trapped” in the complex is formed. The result of the next two reaction steps is the formation of the two HO2● radicals which can react on the triplet energy surface in order to produce O2 and a H2O2.
Downloads: 244






