Chemistry Journal of Moldova
EFFECT OF THE NATURE OF SURFACTANT ON THE REACTIVITY OF C,N-DIPHENYLNITRONE TOWARDS ACRYLONITRILE IN DIFFERENT MICROEMULSION SYSTEMS
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2018 Volume 13, no.2
Pages: 82-88
Kahina Hamza, Abdelkader Touati, Ahmed Ait-Yahia, Michel Baltas, Christiane André Barres, Saâd Moulay
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2018 Volume 13, no.2
Pages: 82-88
acrylonitrile, cycloaddition, isoxazolidine, microemulsion, nitrone.
Full Text (PDF): Download
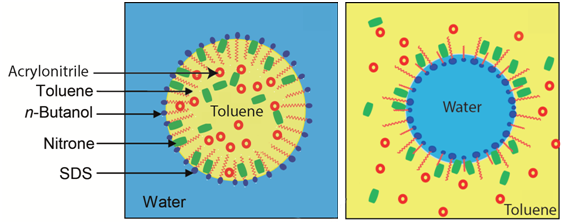
Graphical Abstract: The present work provides an insight into the effect of the nature of surfactant (cationic, anionic), a component of water- and oil-borne microemulsions, on the reaction rate of 1,3-dipolar cycloaddition of C,N-diphenylnitrone with acrylonitrile. The electrostatically attractive character of the cationic surfactant, would bring the reactants closer to each other; hence, a rate enhancement would ensue, particularly within the water-rich zone. Besides the fact that acrylonitrile played a dual role, as a component of the microemulsion and a dipolarphile in the cycloaddition reaction, made the work-up advantageously sound. Additionally, the increase in reagents molar ratio was found to promote higher reactivity.

Downloads: 146






