Chemistry Journal of Moldova
EVALUATING THE METHODS USED FOR THE REGENERATION PROCESS OF COPPER-ZINC SOLUTIONS
Author(s):
Field: Industrial chemistry
Type: Research paper
Issue: 2021 Volume 16, no.1
Pages: 88-98
Vita Datsenko and Vasyl Larin
Field: Industrial chemistry
Type: Research paper
Issue: 2021 Volume 16, no.1
Pages: 88-98
waste, heavy metal, regeneration, reagent method, treatment efficiency.
Full Text (PDF): Download
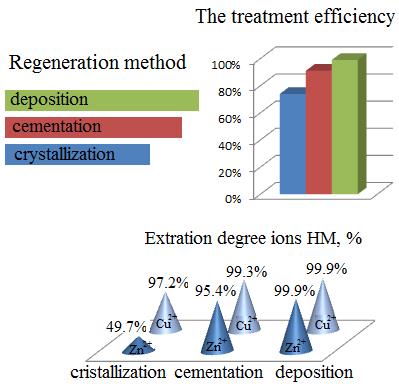
Graphical Abstract: The purpose of this study was to investigate the physicochemical particularities of the regeneration processes that occur in spent sulphate copper-zinc solutions using the reagent methods of crystallization, cementation, and sedimentation. A comparative analysis of the results of the studies of regeneration processes has shown that the content of the extraction of Cu2+ and Zn2+ ions by the crystallization method makes up to 97.2% and 49.7%, respectively; by the contact displacement method to 99.9% and 95.4%, respectively; and by the deposition method it makes up to 99.9% and 99.9%, respectively. The presented study can be used for improvements in the electroplating productivity.
Downloads: 114






