Chemistry Journal of Moldova
SOME PARTICULARITIES OF THE REACTION BETWEEN ANTIOXIDANT PHENOLIC ACIDS AND THE FREE RADICAL ABTS•+: A COMPARATIVE DFT STUDY FOR THE GAS PHASE AND ETHANOL
Author(s):
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2022 Volume 17, no.1
Pages: 24-30
Mikhail Gorbachev, Natalia Gorinchoy, Iolanta Balan
Field: Physical chemistry and chemical physics
Type: Research paper
Issue: 2022 Volume 17, no.1
Pages: 24-30
antioxidant activity, radical cation ABTS•+, food acid, charge transfer complex, DFT calculation.
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: The detailed mechanism of the interaction of the radical cation ABTS•+ with a number of food acids (gallic, ferulic, caffeic, vanillic, cinnamic, syringic, p-coumaric) is revealed by means of the DFT calculations. It is shown that the interaction between the neutral molecules of the studied food acids and ABTS•+ does not lead to any charge transfer from these molecules onto ABTS•+. The almost complete conversion of the ABTS radical cation into its diamagnetic derivative occurs due to the interaction of one of the sulphonic groups of ABTS•+ with the acid anions through the formation of the corresponding intermolecular hydrogen bond.
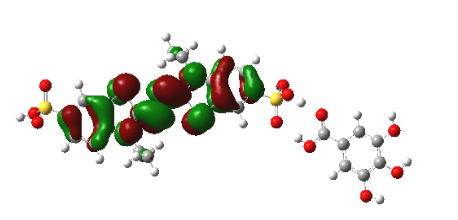
Graphical Abstract: The detailed mechanism of the interaction of the radical cation ABTS•+ with a number of food acids (gallic, ferulic, caffeic, vanillic, cinnamic, syringic, p-coumaric) is revealed by means of the DFT calculations. It is shown that the interaction between the neutral molecules of the studied food acids and ABTS•+ does not lead to any charge transfer from these molecules onto ABTS•+. The almost complete conversion of the ABTS radical cation into its diamagnetic derivative occurs due to the interaction of one of the sulphonic groups of ABTS•+ with the acid anions through the formation of the corresponding intermolecular hydrogen bond.

Downloads: 186






