Chemistry Journal of Moldova
SYNTHESIS AND STRUCTURAL CHARACTERIZATION OF THE TETRANUCLEAR IRON(III) CLUSTER WITH SALICYLIC ACID
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 62-68
Viorina Gorinchoy
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 62-68
homotetranuclear salicylate, iron(III), cluster, IR spectroscopy, X-ray crystal structure.
Full Text (PDF): Download
DOI: https://doi.org/10.19261/cjm.2020.758
Abstract (PDF)
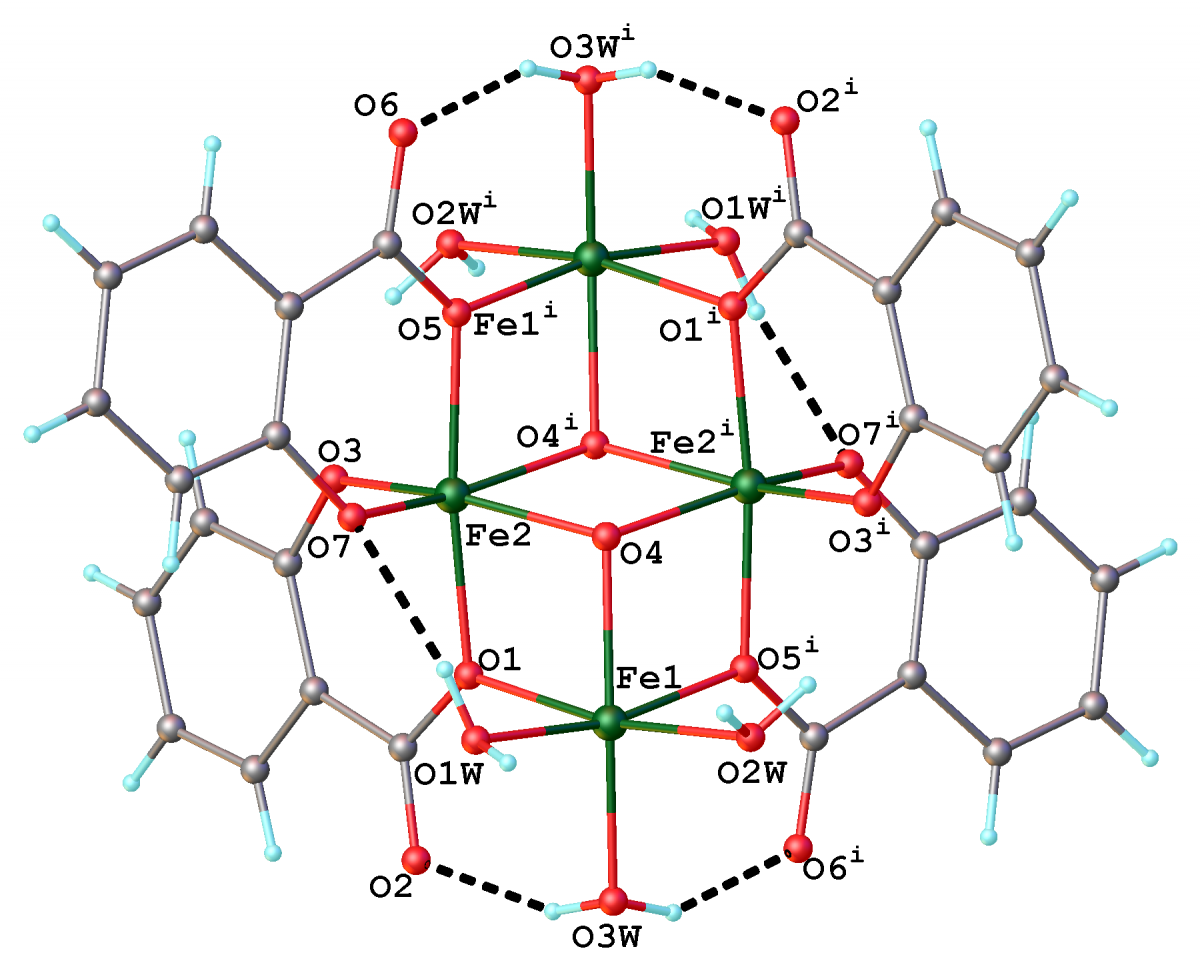
Graphical Abstract: A new tetra-homonuclear iron(III) cluster, [Fe4O2(Sal)4(H2O)6]·4DMA·0.75H2O, where Sal= salicylic acid and DMA= N,N-dimethylacetamide consolidated via two µ3-oxo- and four salicylate-bridges was synthesized and characterized by IR spectroscopic method as well as by single crystal X-ray diffraction analysis. The structure of the obtained tetranuclear compound consists of four FeIII atoms in a “butterfly” arrangement. The coordination sphere of each of the two central FeIII atoms is generated by two μ3-oxo-bridging atoms and four oxygen atoms provided by the tridentate-bridging Sal2- ligands, while the coordination polyhedron of another two iron atoms involve six oxygen atoms from three water molecules, two salicylic and one μ3-oxigen atom. The Fe-O distances within Fe-O-Fe bridge are of 2.102(3) Å (for wing-body) and 2.038(3) Å (for body-body).
Downloads: 140






