Chemistry Journal of Moldova
INFLUENCE OF AROMATIC SUBSTITUENTS ON THE SYNTHESIS OF SCHIFF BASES DERIVED FROM TRANS-(R,R)-DIAMINOCYCLOHEXANE: A SPECTROPHOTOMETRIC AND DFT B3LYP STUDY
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2025 Volume 20, no.2
Pages: 72-83
Lili Dahiana Becerra, Carlos Coy-Barrera, Diego Quiroga
Field: Organic chemistry
Type: Research paper
Issue: 2025 Volume 20, no.2
Pages: 72-83
Schiff base, MW irradiation, substituent effect, spectrophotometric property, DFT calculation.
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2025.1367
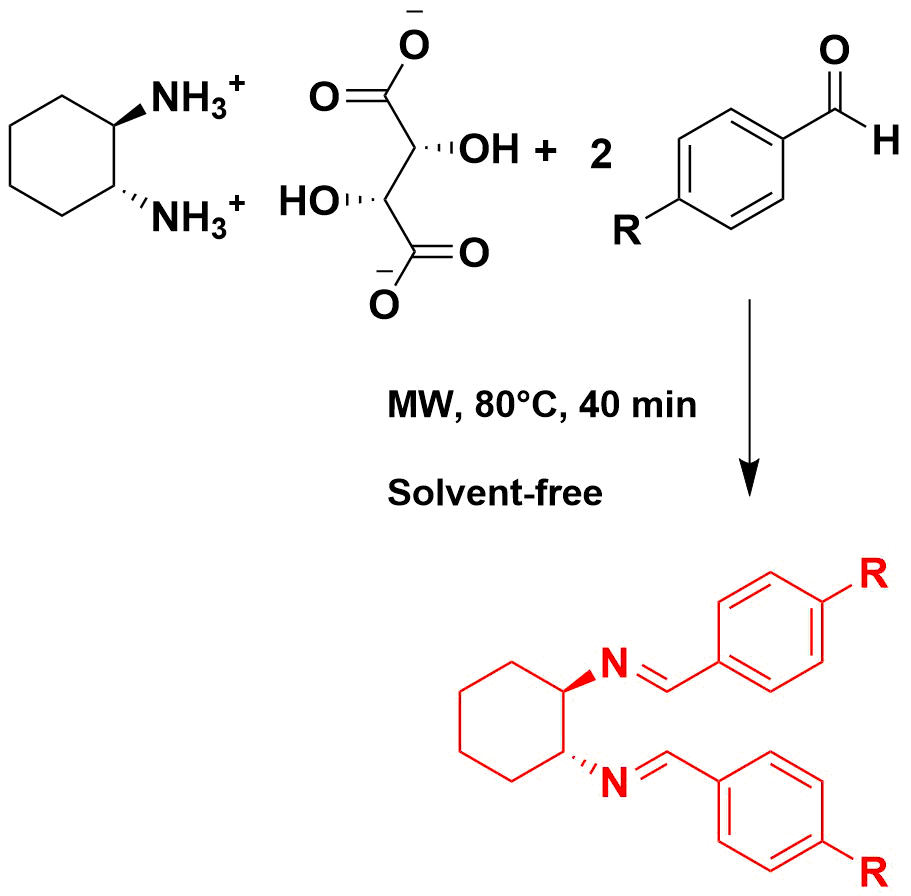
Graphycal Abstract: In this work, the synthesis of Schiff bases derived from trans-(R,R)-diaminocyclohexane by microwave irradiation (MW) is presented. The reaction yields varied between 31% and 69%, being influenced by the electronic nature of the substituents (H, Cl, Br, NO2, MeO, t-BuO, BnO, and 4-(4-Me)PhO) and the reaction temperature. The spectrophotometric properties of the products were investigated by UV-Vis spectrophotometry, revealing bathochromic and hypso-chromic effects attributable to the different substituent groups. These effects were interpreted by DFT calculations with the B3LYP functional at the 6-311G(d,p) level. The results suggest that the electronic properties of the substituents in the para position have a significant impact on the spectroscopic characteristics of the Schiff bases.

Downloads: 24






