Chemistry Journal of Moldova
DESIGN, SYNTHESIS, AND STRUCTURAL STUDY OF MONO- AND POLYNUCLEAR Cu(II) IMINODIACETATE COMPLEXES
Author(s):
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2025 Volume 20, no.2
Pages: 15-24
Dumitru Ureche, Pavlina Bourosh, Ion Bulhac
Field: Inorganic and coordination chemistry
Type: Research paper
Issue: 2025 Volume 20, no.2
Pages: 15-24
coordination compound, Cu(II), iminodiacetic acid, dioxime, X-ray study.
Full Text (PDF): Download
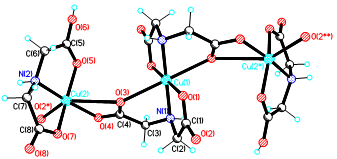
Graphycal Abstract: The synthesis of three structurally distinct copper(II) coordination compounds with iminodiacetic acid (H₂IDA) as ligand was achieved by varying the reaction pH. Under neutral conditions (pH 6–6.5), compound 1 formed as a two-dimensional ionic coordination polymer, {[NH₂(CH₃)₂]₂[Cu₃(IDA)₄]·1.75H₂O}ₙ, consisting of layered [Cu₃(IDA)₄]²ₙ⁻ anions stabilized by hydrogen-bonding networks. Under basic conditions (pH 8–8.5), compound 2 was obtained as a neutral 2D molecular polymer, {[Cu₃(IDA)₂(IDAH)₂]·5H₂O}ₙ, built from trinuclear copper units bridged by bi- and monodeprotonated ligands. Under acidic conditions (pH 3), compound 3, ((CH₃)₂OH)₂[Cu(IDA)₂]·[Cu(IDAH)₂], was isolated as an ionic structure containing neutral and anionic mononuclear complexes charge-balanced by protonated dimethylether cations.

Downloads: 23






