Chemistry Journal of Moldova
SYNTHESIS, CHARACTERIZATION, HIRSHFELD AND ADMET ESTIMATION STUDIES OF NOVEL 3-(2,4,6-TRIMETHYL-PHENYLAMINO)-BUT-2-ENOATE
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.2
Pages: 83-92
Mohamed Loughzail, Koffi Senam Etsè, Zaragoza Verez Guillermo, Rachid Touzani, Anna Moliterni, Mohamed Anouar Harrad, Abdessamad Tounsi
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.2
Pages: 83-92
β-enaminoester; X-ray diffraction, monoclinic space, Hirshfeld surface, ADMET-Tox.
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2024.1220
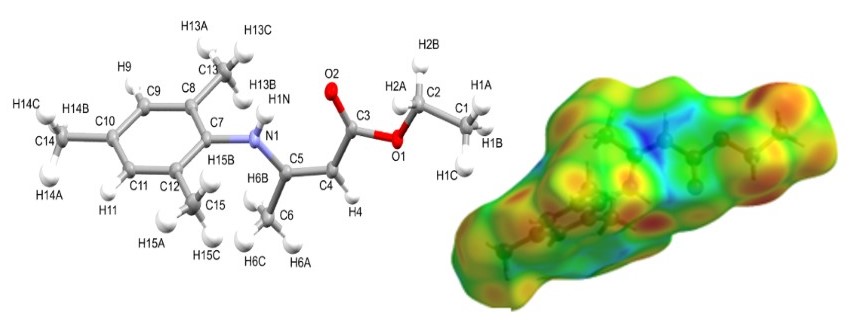
Graphycal Abstract: The compound 3-(2,4,6-timethyl-phenylamino)-but-2-enoate was obtained by the condensation reaction of ethyl acetoacetate and 2,4,6-trimethyl-phenylamine. X-ray structural analysis identified the structure of the synthesized β-enaminoester, NMR spectroscopy complemented it, and the structure stabilised by intramolecular interactions. The intermolecular contacts were further analysed by the mapping of contacts descriptors dnorm, de, di, the shape-by-shape index and surface property by electrostatic potential mapped on the Hirshfeld surface (HS). Global reactivity factors such as electronegativity, chemical hardness, potential, and softness were calculated using density functional theory. The effects of the molecular environment were accessed by analysing the electrostatic potentials surface mapped over the HS and the 3D-topology of energy frameworks. As a potential bioactive molecule, the physicochemical and ADME-Tox predictions were performed suggesting that title compound could be considered a promising drug candidate.
Downloads: 112






