Chemistry Journal of Moldova
Analytical chemistry
Author(s):
Field: Analytical chemistry
Type: Research paper
Issue: 2025 Volume 20, no.1
Pages: 7-16
Abdelghani Mahmoudi and Silvia De Francia
Field: Analytical chemistry
Type: Research paper
Issue: 2025 Volume 20, no.1
Pages: 7-16
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2025.1312
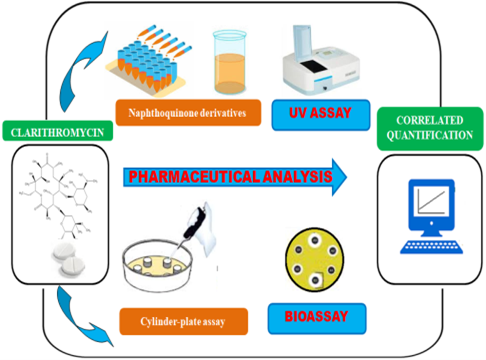
Graphycal Abstract: Novel spectrophotometric method and bioassay using the Bacillus subtilis strain were developed for clarithromycin analysis. Experimental conditions were optimised and validated according to ICH guidelines. A comparative study was established, and the methods were successfully applied for the quantification of clarithromycin in solid dosage forms and can be used for pharmaceutical purposes.

Downloads: 67
Author(s):
Field: Analytical chemistry
Type: Research paper
Issue: 2024 Volume 19, no.1
Pages: 37-46
Abdelghani Mahmoudi and Ann Van Schepdael
Field: Analytical chemistry
Type: Research paper
Issue: 2024 Volume 19, no.1
Pages: 37-46
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2024.1165
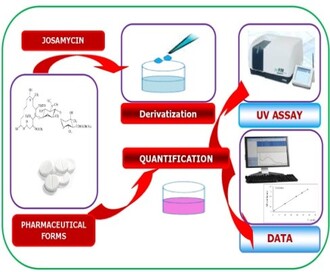
Graphical Abstract: The developed spectrophotometric methods were based on charge transfer reactions of naphthoquinones with Josamycin. Methods validity was tested and the results were in accordance with ICH guidelines. Procedures showed successful adaptability for an easy analysis of this macrolide in various dosage forms and can be used for quality control purposes.
Downloads: 600
Author(s):
Field: Analytical chemistry
Type: Research paper
Issue: 2024 Volume 19, no.1
Pages: 29-36
Crina Vicol, Alexandra Sârghi, Adrian Fifere, Gheorghe Duca
Field: Analytical chemistry
Type: Research paper
Issue: 2024 Volume 19, no.1
Pages: 29-36
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2024.1190
Abstract (PDF)
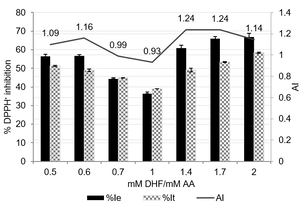
Graphical Abstract: The study presents data on the antioxidant interaction between ascorbic and dihydroxyfumaric acids determined via DPPH method, by applying EPR spectroscopy. The type of antioxidant interaction is dependent on the concentration ratio of the ascorbic and dihydroxyfumaric acids, thus, at the mM DHF/mM AA ratios of 1.4 and 1.7 the highest synergistic effects of 1.24 have been noticed, but at the mM DHF/mM AA ratio of 1 – an antagonistic effect of 0.93 was registered.
Downloads: 101
Author(s):
Field: Analytical chemistry
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 8-21
Imeda Rubashvili, Natela Karukhnishvili, Khatuna Makharadze
Field: Analytical chemistry
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 8-21
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: A new, rapid and selective, HPLC method for simultaneous quantitative estimation of lisinopril and hydrochlorothiazide residues and sampling procedures from pharmaceutical manufacturing equipment surfaces were developed and validated. The sampling procedures have a good recovery (>80%). The limit of quantitation of the HPLC method - 0.155 µg/mL and 0.025 µg/mL for lisinopril and hydrochlorothiazide, respectively.
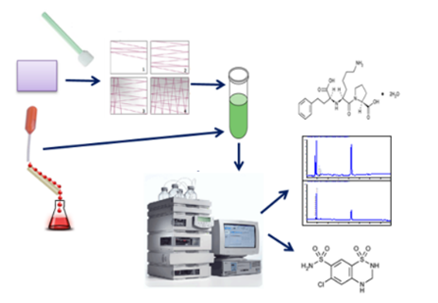
Graphical Abstract: A new, rapid and selective, HPLC method for simultaneous quantitative estimation of lisinopril and hydrochlorothiazide residues and sampling procedures from pharmaceutical manufacturing equipment surfaces were developed and validated. The sampling procedures have a good recovery (>80%). The limit of quantitation of the HPLC method - 0.155 µg/mL and 0.025 µg/mL for lisinopril and hydrochlorothiazide, respectively.

Downloads: 204
Author(s):
Field: Analytical chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 56-61
Livia Uncu, Elena Donici, Vladimir Valica, Oxana Vîslouh, Veaceslav Gonciar, Sergiu Parii
Field: Analytical chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 56-61
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: A simple, precise and accurate UV-Vis spectrophotometric method has been developed and validated for the estimation of ciprofloxacin hydrochloride from combination ear drops with basil oil (Ocimum basilicum). The results of the validation of the method demonstrate that the developed method is simple, rapid, accurate and robust over the concentration range 2-10 μg/mL of ciprofloxacin hydrochloride in combination with volatile basil oil.
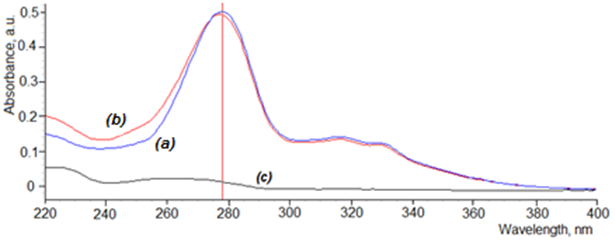
Graphical Abstract: A simple, precise and accurate UV-Vis spectrophotometric method has been developed and validated for the estimation of ciprofloxacin hydrochloride from combination ear drops with basil oil (Ocimum basilicum). The results of the validation of the method demonstrate that the developed method is simple, rapid, accurate and robust over the concentration range 2-10 μg/mL of ciprofloxacin hydrochloride in combination with volatile basil oil.

Downloads: 178
Author(s):
Field: Analytical chemistry
Type: Short communication
Issue: 2018 Volume 13, no.1
Pages: 111-116
Gheorghe Duca, Lilia Anghel, Raul Victor Erhan
Field: Analytical chemistry
Type: Short communication
Issue: 2018 Volume 13, no.1
Pages: 111-116
Full Text (PDF): Download
Graphical Abstract: In this work, Fourier transform infrared spectroscopy was used to highlight the structural differences between the human lactoferrin and human serum transferrin. The results clearly show the structural differences of human lactoferrin and human serum transferrin. The second derivative analysis of the FTIR spectra allows the direct identification of secondary structure components of the human lactoferrin and human serum transferrin.
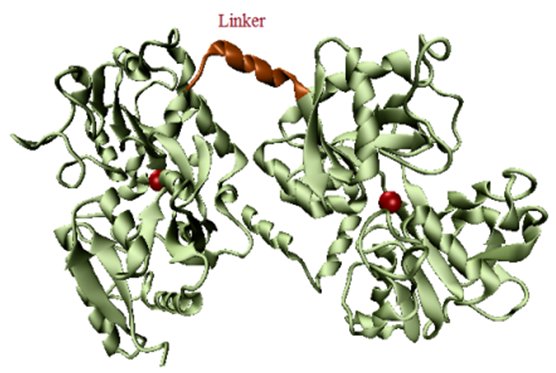

Downloads: 205
Author(s):
Field: Analytical chemistry
Type: Review
Issue: 2015 Volume 10, no.2
Pages: 8-25
Oxana Spinu, Igor Povar
Field: Analytical chemistry
Type: Review
Issue: 2015 Volume 10, no.2
Pages: 8-25
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2015.10(2).01
Graphical Abstract: The quantitative basis of the theory of buffer properties for two-phase acid-base buffer systems and for multicomponent heterogeneous systems has been derived. The analytical equations with respect to all components for diverse multicomponent systems were deduced. It has been established, that the buffer capacities of components are mutually proportional.
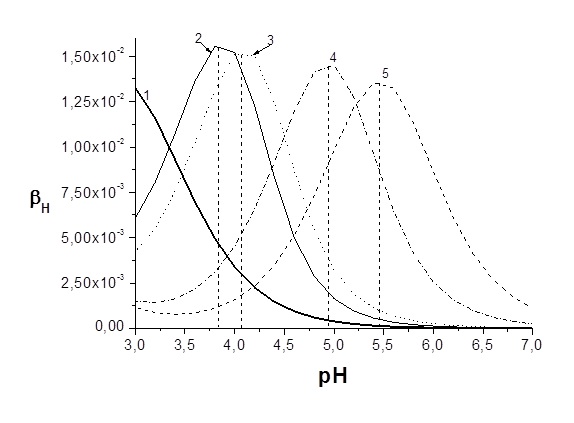
Downloads: 80
Author(s):
Field: Analytical chemistry
Type: Short communication
Issue: 2015 Volume 10, no.1
Pages: 113-115
Diana Shepel, Tatiana Goreacioc, Tudor Lupascu, Mihail Filippov, Maria Rusu
Field: Analytical chemistry
Type: Short communication
Issue: 2015 Volume 10, no.1
Pages: 113-115
Full Text (PDF): Download
Abstract (PDF)
DOI: dx.doi.org/10.19261/cjm.2015.10(1).16
Graphical Abstract: This communication is devoted to the elaboration of a new optimal technique of infrared spectra registration of activated carbons in potassium bromide pellets. Authors investigated the dependence of the intensity of the least overlapping infrared bands of activated carbons on the conditions of preparation, recording of the spectrum, and the degree of homogenization with potassium bromide.
Downloads: 56
DOI: dx.doi.org/10.19261/cjm.2015.10(1).16
Graphical Abstract: This communication is devoted to the elaboration of a new optimal technique of infrared spectra registration of activated carbons in potassium bromide pellets. Authors investigated the dependence of the intensity of the least overlapping infrared bands of activated carbons on the conditions of preparation, recording of the spectrum, and the degree of homogenization with potassium bromide.

Downloads: 56
Author(s):
Field: Analytical chemistry
Type: Research paper
Issue: 2014 Volume 9, no.2
Pages: 8-13
Maria Sandu, Tudor Lupascu, Anatol Tarita, Tatiana Goreacioc, Sergiu Turcan, Elena Mosanu
Field: Analytical chemistry
Type: Research paper
Issue: 2014 Volume 9, no.2
Pages: 8-13
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2014.09(2).01
Graphical Abstract: The study relates to determination of nitrate in presence of nitrite in water and can be used in the quality monitoring of natural water (surface and groundwater), drinking water, water from fish farms and public aquaria where autonomous filters is used. The nature and quantity of reagents used have insignificant impact on natural waters and sewages. According to the investigation, the method includes the removal of nitrite from the solution/water with sulfaminic acid, the nitrate ion reduction to nitrite using a reducing mixture that contains Na2SO4 and zinc dust in ratio of 100:5 and determining the nitrite with the Griess reagent.
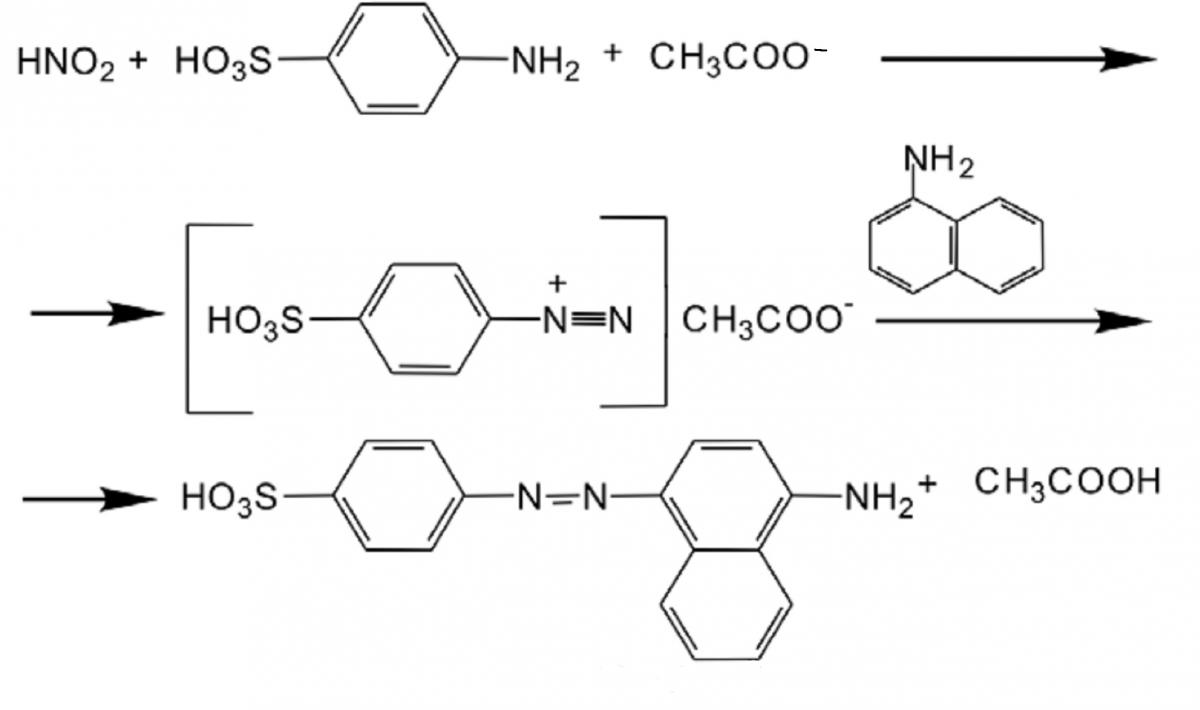
Downloads: 198
Author(s):
Field: Analytical chemistry
Type: Research paper
Issue: 2014 Volume 9, no.2
Pages: 14-18
Raluca Anamaria Cristache, Ana Maria Budu, Petronela Spiridon, Viorica Vasilache, Ion Sandu
Field: Analytical chemistry
Type: Research paper
Issue: 2014 Volume 9, no.2
Pages: 14-18
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2014.09(2).02
Graphical Abstract: The authentication of cultural heritage assets is a complex process of scientific investigation which regards obtaining information about: author (painter), the period when it was made, the owners and other data related to the main contexts of their evolution in time (routes travelled). This work presents a comparative study of the paper used in the preparation layer of three icons painted in Russian style. As an analysis techniques were used the micro and macro photography assisted by Optical Microscopy (OM), SEM-EDX and micro-FTIR.
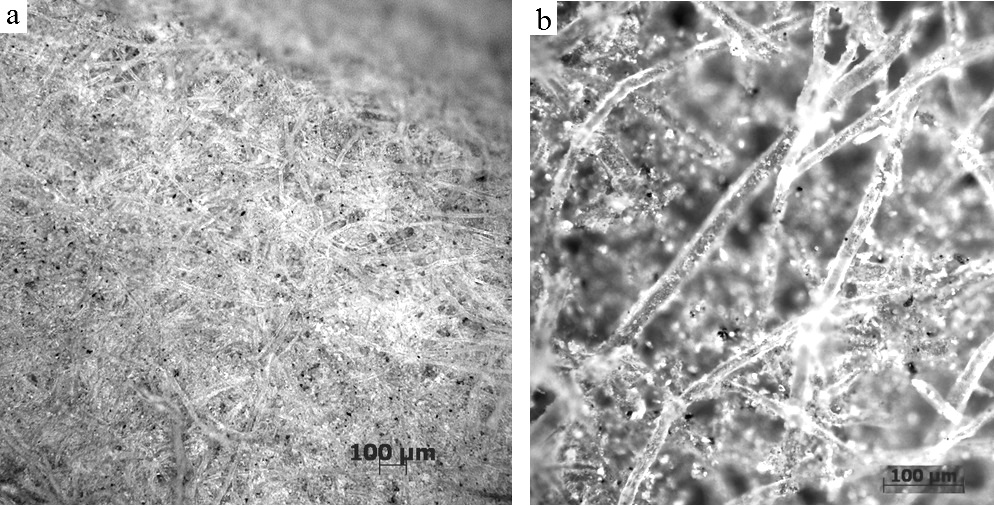
Downloads: 36






