Chemistry Journal of Moldova
Natural product chemistry and synthesis
Author(s):
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2016 Volume 11, no.2
Pages: 105-108
Ion Dragalin, Aculina Aricu, Nina Ciocarlan, Alexandru Ciocarlan, Victoria Codita
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2016 Volume 11, no.2
Pages: 105-108
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2016.11(2).06
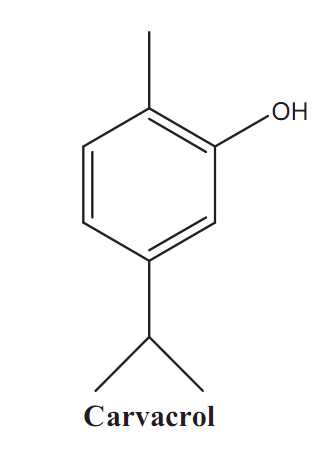
Graphical Abstract: For the first time the results of GC-MS analysis of Satureja subspicata L. oil of Moldovan origin are reported. The chemical profile includes forty-four constituents and consists mostly (97.86%) of phenolic monoterpenes, monoterpene hydrocarbons, bicyclic sesquiterpenes and their oxygenated derivatives. A substantial quantitative and qualitative chemical differentiation of S. subspicata oil of Moldovan origin and reported oil of Croatian origin were found. The essential oil of S. subspicata L. plants cultivated in Republic of Moldova belongs to the carvacrol chemotype.

Downloads: 85
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2016 Volume 11, no.1
Pages: 50-54
Elena Secara
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2016 Volume 11, no.1
Pages: 50-54
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2016.11(1).06
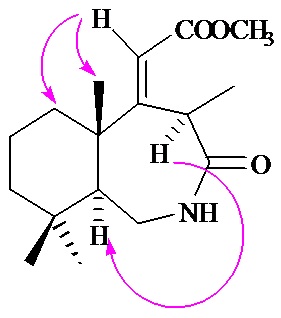
Graphical Abstract: Synthesis of new drimane and homodrimane lactams, derivatives of octahydro-1H-benzo[d]azepine and octahydro-1H-benzo[c]azepine, from norambreinolide is reported. These compounds were prepared by Beckmann rearrangement of the corresponding ketooximes.
The article is Open Access by CC-BY 4.0 License
Cite this article as: E. Secara. Synthesis of New Drimane and Homodrimane Lactams by Beckmann Rearrangement of Some Ketoximes. Chemistry Journal of Moldova, 2016, 11(1), pp. 50-64. DOI: dx.doi.org/10.19261/cjm.2016.11(1).06
Article Source: Synthesis of New Drimane and Homodrimane Lactams by Beckmann Rearrangement of Some Ketoximes.
Download article metadata: CrossRef BibTeX CERIF Google Scholar DataCite Dublin Core
Downloads: 91
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2015 Volume 10, no.2
Pages: 54-57
Ion Dragalin, Olga Morarescu, Maria Sedcenco, Radu Marin Rosca
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2015 Volume 10, no.2
Pages: 54-57
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2015.10(2).06
Graphical Abstract: The accumulated as production waste fat from Faraon quail breeds has been investigated for the first time by using GC-MS technique, preventively converting it via methanolysis to fatty acid methyl esters. The test results, regarding the content of unsaturated fatty acids having a favorable to human body cis-configuration (77.8%), confirm their nutritional value and the possibility of using this fat in cosmetic, pharmaceutical and food industries.
Downloads: 71
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2015 Volume 10, no.2
Pages: 58-61
Lidia Lungu
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2015 Volume 10, no.2
Pages: 58-61
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2015.10(2).07
Graphical Abstract: The synthesis of new nitrogen-containing drimane and homodrimane sesquiterpenoids in cycle B is reported. A comparative study of the microwave (MW) assisted synthesis of drimenone versus classical conditions has been done. The drimanic and homodrimanic oximes were prepared on the base of ketones derived from commercially available sclareolide. The drimanic amine was obtained by reduction of corresponding oxime with LiAlH4. The structure of novel compounds was confirmed using IR, 1H and 13C NMR analyses.
Downloads: 76
Author(s):
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2015 Volume 10, no.1
Pages: 9-19
Olga Morarescu
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2015 Volume 10, no.1
Pages: 9-19
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2015.10(1).01
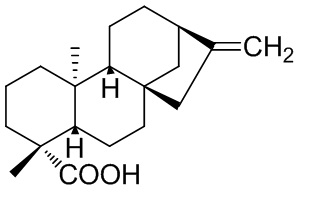
Graphical Abstract: This review presents a synthetic transformations of ent-kaurane diterpenes, covering various aspects of the chemical and microbiologically transformations of native ent-kaur-16-en-19-oic acid, namely, its reactions via COOH groups, double bonds and rearrangements of the carbon skeleton, what we offer a wide range of natural and synthetic derivatives potentially biologically actives and convenient synthon for their synthesis.
Downloads: 460
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2015 Volume 10, no.1
Pages: 57-63
Natalia Mashcenko, Angela Gurev, Galina Lupascu, Elena Gorincioi
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2015 Volume 10, no.1
Pages: 57-63
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2015.10(1).08
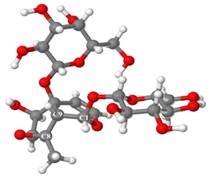
Graphical Abstract: The paper relates on the discovered bioactivity of the iridoid glycosides extract (IGE) from Linaria genistifolia (L.) Mill, namely its stimulating influence on the resistance of the winter wheat Odesschi 51 plant to the caused by the F. oxysporum and H. avenae pathogenic fungi root rot. 1H and 13C NMR characteristics of 5-O-allosylantirrinoside in Py-d5 are for the first time presented. Structures of two conformers of the IGE main component, 5-O-allosylantirrinoside in D2O and Py-d5 solutions are proposed, based on the experimental NMR evidence and molecular modeling studies.
Downloads: 62
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2013 Volume 8, no.2
Pages: 90-93
Olga Morarescu, Marina Grinco, Ion Dragalin, Veaceslav Kulciţki, Nicon Ungur
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2013 Volume 8, no.2
Pages: 90-93
Full Text (PDF): Download
Author(s):
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2013 Volume 8, no.2
Pages: 94-100
Marina Grinco, Veaceslav Kulciţki, Pavel F. Vlad, Alic Barba, Elena Gorincioi, Nicon Ungur
Field: Natural product chemistry and synthesis
Type: Research paper
Issue: 2013 Volume 8, no.2
Pages: 94-100
Full Text (PDF): Download
Author(s):
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2012 Volume 7, no.2
Pages: 147-148
S. Kovalskaya, N. Kozlov, A. Aricu, V. Kulcitki, N. Ungur
Field: Natural product chemistry and synthesis
Type: Short communication
Issue: 2012 Volume 7, no.2
Pages: 147-148
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2012.07(2).01
Graphical Abstract: The main products of sclareol (1) Ritter’s reaction in mild conditions are (8R,13R)-Labd-14(15)-en-8,13-diacetamide (2) (8R,13S)-Labd-14(15)-en-8,13-diacetamide (3) stereoisomeric on C13 atom and having unrearranged native diol skeleton. We present in the current communication the results of sclareol converting (1) into nitrogen-containing labdanes
in the Ritter’s reaction conditions.
Downloads: 69
Author(s):
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2012 Volume 7, no.2
Pages: 57-66
L. Zadorojnai, A. Zadorojnai
Field: Natural product chemistry and synthesis
Type: Review
Issue: 2012 Volume 7, no.2
Pages: 57-66
Full Text (PDF): Download
DOI: dx.doi.org/10.19261/cjm.2012.07(2).13
Graphical Abstract: Properties and methods for obtaining hyaluronic acid and its derivatives from raw material of animal origin are reviewed.
The importance and practical application of hyaluronic acid in various fields are discussed. This article is an extended abstract of a communication presented at the Conference Ecological Chemistry 2012.
Downloads: 41






