Chemistry Journal of Moldova
Organic chemistry
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 99-104
Kabeer Ahmed Shaikh and Uddhav Nivrutti Chaudhar
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 99-104
Full Text (PDF): Download
Abstract (PDF)
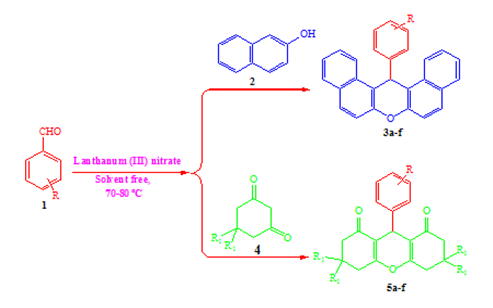
Graphical Abstract: The present paper shows that lanthanum(III) nitrate hexahydrate can be used as mild and environment friendly homogeneous catalyst for an efficient one-pot multi-component synthesis of biologically active 1,8-dioxo-octahydroxanthene and 14H-dibenzo[a,j]xanthene derivatives. The solvent free condensation reaction of aromatic aldehydes and dimedone or β-naphthol was carried out at 70-80ºC during 10-30 min. The advantages of this eco-friendly synthesis route are numerous, and include the use of an inexpensive catalyst, high to excellent yield, short reaction time and high catalytic activity that can make this method an interesting alternative to multi-step approaches.
Downloads: 143
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 88-98
Roman Rusnac, Maria Botnaru, Nicanor Barba, Peter Petrenko, Yurii Chumakov, Aurelian Gulea
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.2
Pages: 88-98
Full Text (PDF): Download
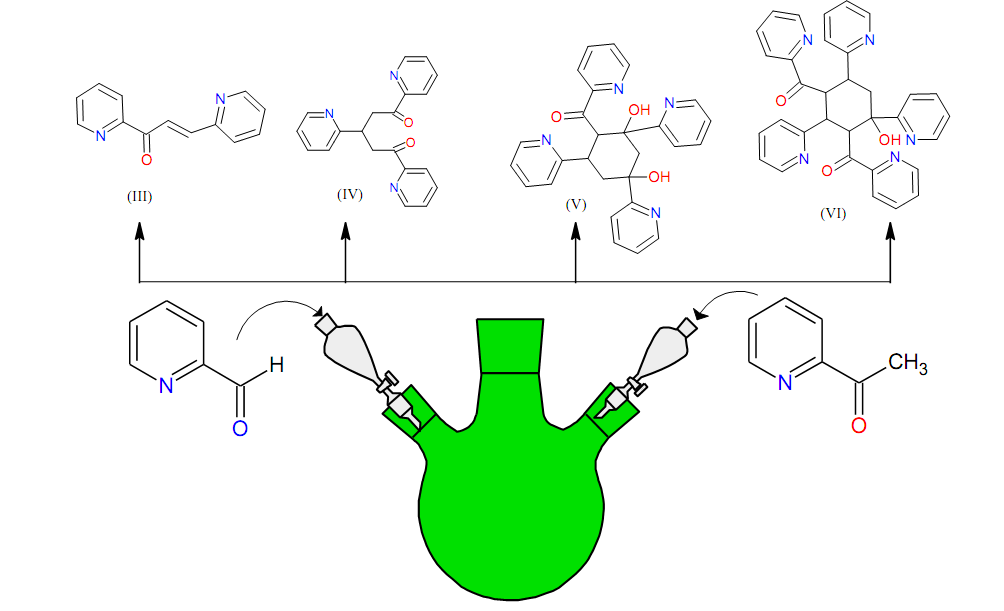
Graphical Abstract: The research is devoted to the study of unexpected products that formed as a result of the condensation reaction between 2-acetylpyridine and 2-formylpyridine under the Claisen-Schmidt reaction conditions. As a result, a sequence of reactions leading to the following compounds has been proposed: 1,3-bis (pyridin-2-yl) prop-2-en-1-one (3); 1,3,5-tri (pyridin-2-yl) pentane-1,5-dione (4); (2,4-dihydroxy-2,4,6-tri(pyridin-2-yl)cyclohexyl)(pyridin-2-yl)methanone (5) and (4-hydroxy-2,4,6-tri(pyridin-2-yl)cyclohexane-1,3-diyl)bis(pyridin-2-ylmethanone) (6) as well as 2-formylpyridine (1) and 2-acetylpyridine (2). The plausible mechanism of these chemical transformations has been proposed. All the obtained compounds demonstrate moderate antimicrobial, antifungal and antioxidant activity.

Downloads: 178
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 75-85
Lauro Figueroa, Alejandra Garcimarero, Rolando Garcia, Francisco Diaz, Marcela Rosas, Virginia Mateu, Maria Lopez, Lenin Hau, Tomas Lopez, Abelardo Camacho, Yaritza Borges
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 75-85
Full Text (PDF): Download
Abstract (PDF)
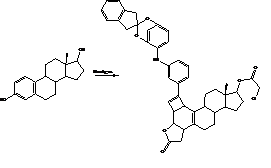
Graphical Abstract: This study describes the preparation of two bicyclo[3.3.3]nonane-steroid derivatives from either estradiol or estrone using some reactions such as etherification, addition, nucleophilic substitution and cyclization. The chemical structure was evaluated through NMR spectroscopic analysis. The results showed higher yield for 11 compared with 12. It is noteworthy that the reagents used in this investigation are not expensive and do not require special conditions for handling.
Downloads: 373
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 86-94
Hajer Hrichi, Nadia Ali Ahmed Elkanzi, Rania Badawy Bakr
Field: Organic chemistry
Type: Research paper
Issue: 2020 Volume 15, no.1
Pages: 86-94
Full Text (PDF): Download
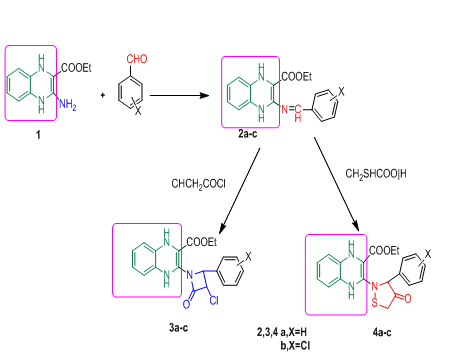
Graphical Abstract: A series of novel isolated β-lactams 3a-c and thiazolidinone derivatives 4a-c were successfully synthesized from reactions of new Schiff's bases 2a-c with chloroacetyl chloride and thioglycolic acid. The antimicrobial activity of the obtained compounds was assessed in vitro against gram-positive Staphylococcus aureus and gram-negative Escherichia coli bacteria and Aspergillus flavus and Candida albicans fungi. Furthermore, a molecular docking study was carried out and the results indicated that compounds 3b and 4b displayed comparable binding affinity scores as that of glutamate. These two compounds are promising candidates as antibacterial and antifungal agents that would deserve further investigations.

Downloads: 246
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 90-96
Jumina Jumina, Rizky Woro Styaningrum, Dwi Siswanta, Sugeng Triono, Yoga Priastomo, Harizal Harizal, Eti Nurwening Sholikhah, Abdul Karim Zulkarnain
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 90-96
Full Text (PDF): Download
DOI: http://dx.doi.org/10.19261/cjm.2019.624
Abstract (PDF)
Graphical Abstract: Four chalcone derivatives were synthesized and pre-evaluated as broad-spectrum UV protector. Chalcones 1-4 showed a wide range of UV absorbance values and moderate molar absorptivity values. Chalcones 3 and 4 showed better photostability than chalcones 1 and 2 because the lowering of their absorbance was smaller and slower under UVB irradiation. A combination of the spectra of chalcone derivatives 1-4 indicated that a formulation containing all four will provide a broad-spectrum sunscreen protecting the skin from UVA and UVB.
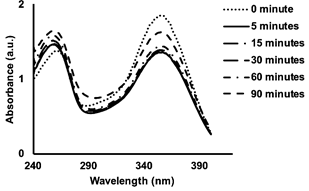
Graphical Abstract: Four chalcone derivatives were synthesized and pre-evaluated as broad-spectrum UV protector. Chalcones 1-4 showed a wide range of UV absorbance values and moderate molar absorptivity values. Chalcones 3 and 4 showed better photostability than chalcones 1 and 2 because the lowering of their absorbance was smaller and slower under UVB irradiation. A combination of the spectra of chalcone derivatives 1-4 indicated that a formulation containing all four will provide a broad-spectrum sunscreen protecting the skin from UVA and UVB.

Downloads: 270
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 105-116
Nadia Ali Ahmed Elkanzi, Amira Atef Ghoneim, Hajer Hrichi
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 105-116
Full Text (PDF): Download
DOI: http://dx.doi.org/10.19261/cjm.2019.638
Abstract (PDF)
Graphical Abstract: In this study, novel pyrazole, imidazole, pyrimidine derivatives bearing imidazo[4,5-b]indol moiety were successfully synthesized and their chemical structures were identified and confirmed by different spectral techniques. All the synthesized compounds were tested against four bacterial strains (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa) and two fungi (Aspergillus flavus and Candida albicans). The obtained results show that the synthesized compounds could find fruitful applications as antibacterial and antifungal agents in pharmaceutical chemistry.
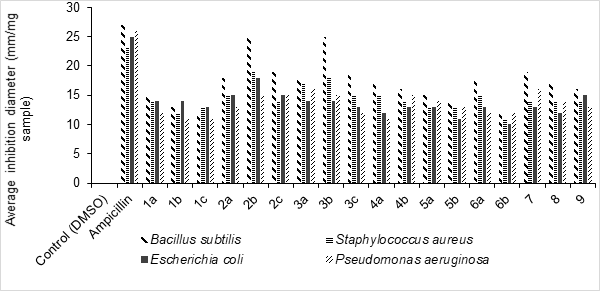
Graphical Abstract: In this study, novel pyrazole, imidazole, pyrimidine derivatives bearing imidazo[4,5-b]indol moiety were successfully synthesized and their chemical structures were identified and confirmed by different spectral techniques. All the synthesized compounds were tested against four bacterial strains (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa) and two fungi (Aspergillus flavus and Candida albicans). The obtained results show that the synthesized compounds could find fruitful applications as antibacterial and antifungal agents in pharmaceutical chemistry.

Downloads: 213
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 97-104
Maryam Shokoohian, Nourallah Hazeri, MalekTaher Maghsoodlou, Mojtaba Lashkari
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.2
Pages: 97-104
Full Text (PDF): Download
Graphical Abstract: 4-(Dimethylamino)pyridine was found to be an efficient homogenous catalyst for one-pot multi-component reactions between hydrazine monohydrate, ethyl cyanoacetate, ketone, and malononitrile for the synthesis of 1,6-diamino-2-oxo-1,2,3,4-tetrahydropyridine-3,5-dicarbonitrile derivatives using ultrasonication at room temperature in ethanol solution within 35-50 min with yields of over 90%.
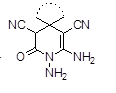
Downloads: 137
Author(s):
Field: Organic chemistry
Type: Review
Issue: 2019 Volume 14, no.2
Pages: 32-55
Serghei Curlat
Field: Organic chemistry
Type: Review
Issue: 2019 Volume 14, no.2
Pages: 32-55
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: This review presents last decade and some past especially relevant studies in the field of (+)-3-carene synthetic transformations. This paper discusses exclusively the transformations of (+)-3-carene, proceeding with the retention of the native bicyclic carbon skeleton. The data concerning the features of epoxidation and oxidation reactions of (+)-3-carene, the synthesis of sulphur- and selenium-containing derivatives and their use in asymmetric synthesis are given. It also describes methods for producing amino derivatives of (+)-3-carene, substituted heterocycles based on it, reactions for the preparation of aziridines, azido-alcohols and azidoamines, as well as chiral phosphites as bidentate ligands.
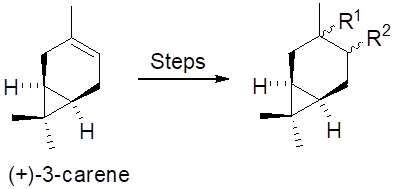
Graphical Abstract: This review presents last decade and some past especially relevant studies in the field of (+)-3-carene synthetic transformations. This paper discusses exclusively the transformations of (+)-3-carene, proceeding with the retention of the native bicyclic carbon skeleton. The data concerning the features of epoxidation and oxidation reactions of (+)-3-carene, the synthesis of sulphur- and selenium-containing derivatives and their use in asymmetric synthesis are given. It also describes methods for producing amino derivatives of (+)-3-carene, substituted heterocycles based on it, reactions for the preparation of aziridines, azido-alcohols and azidoamines, as well as chiral phosphites as bidentate ligands.

Downloads: 154
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 68-76
Emmy Yuanita, Harno Dwi Pranowo, Mustofa Mustofa, Respati Tri Swasono, Jufrizal Syahri, Jumina Jumina
Field: Organic chemistry
Type: Research paper
Issue: 2019 Volume 14, no.1
Pages: 68-76
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: In this study, the chloro-substituted hydroxyxanthones were prepared by cyclodehydration of acid derivatives and substituted phenol in the presence of Eaton reagent, followed by halogenations step to electrophilic substitution of chlorine in a moderate yield. The in vitro anticancer activity study on various cell lines revealed that the chloro functional group increases the anticancer activity of the hydroxyxanthone derivatives. The molecular docking study showed that there was a binding interaction between chloro-hydroxyxanthone and the amino acid residues such as Asp810, Cys809, Ile789, His790, and Leu644 of protein tyrosine kinase receptor.
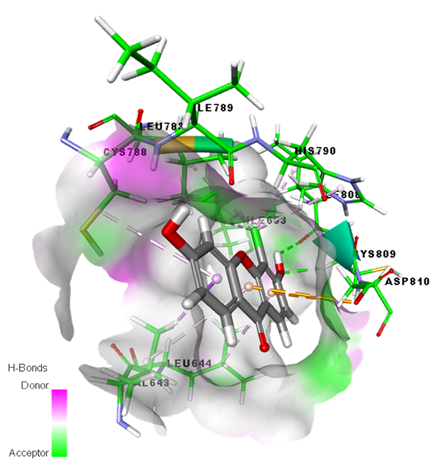
Graphical Abstract: In this study, the chloro-substituted hydroxyxanthones were prepared by cyclodehydration of acid derivatives and substituted phenol in the presence of Eaton reagent, followed by halogenations step to electrophilic substitution of chlorine in a moderate yield. The in vitro anticancer activity study on various cell lines revealed that the chloro functional group increases the anticancer activity of the hydroxyxanthone derivatives. The molecular docking study showed that there was a binding interaction between chloro-hydroxyxanthone and the amino acid residues such as Asp810, Cys809, Ile789, His790, and Leu644 of protein tyrosine kinase receptor.

Downloads: 615
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2018 Volume 13, no.1
Pages: 74-86
Farzaneh Mohamadpour, Malek Taher Maghsoodlou, Mojtaba Lashkari, Reza Heydari, Nourallah Hazeri
Field: Organic chemistry
Type: Research paper
Issue: 2018 Volume 13, no.1
Pages: 74-86
Full Text (PDF): Download
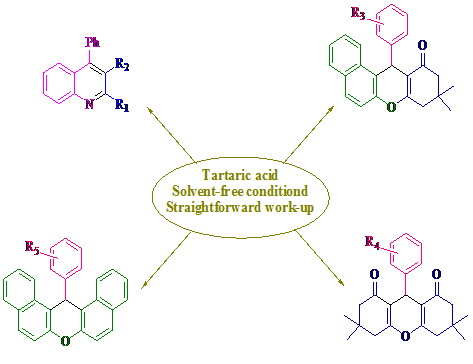
Graphical Abstract: This method reported the use of tartaric acid as a green and highly efficient catalyst for the convenient synthesis of polysubstituted quinolines and xanthenes derivatives in excellent yields under solvent-free conditions. The main advantages of this one-pot procedure are the green and economic availability of the catalyst, simple experimental and work-up procedures.

Downloads: 217






