Chemistry Journal of Moldova
Organic chemistry
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2025 Volume 20, no.2
Pages: 72-83
Lili Dahiana Becerra, Carlos Coy-Barrera, Diego Quiroga
Field: Organic chemistry
Type: Research paper
Issue: 2025 Volume 20, no.2
Pages: 72-83
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2025.1367
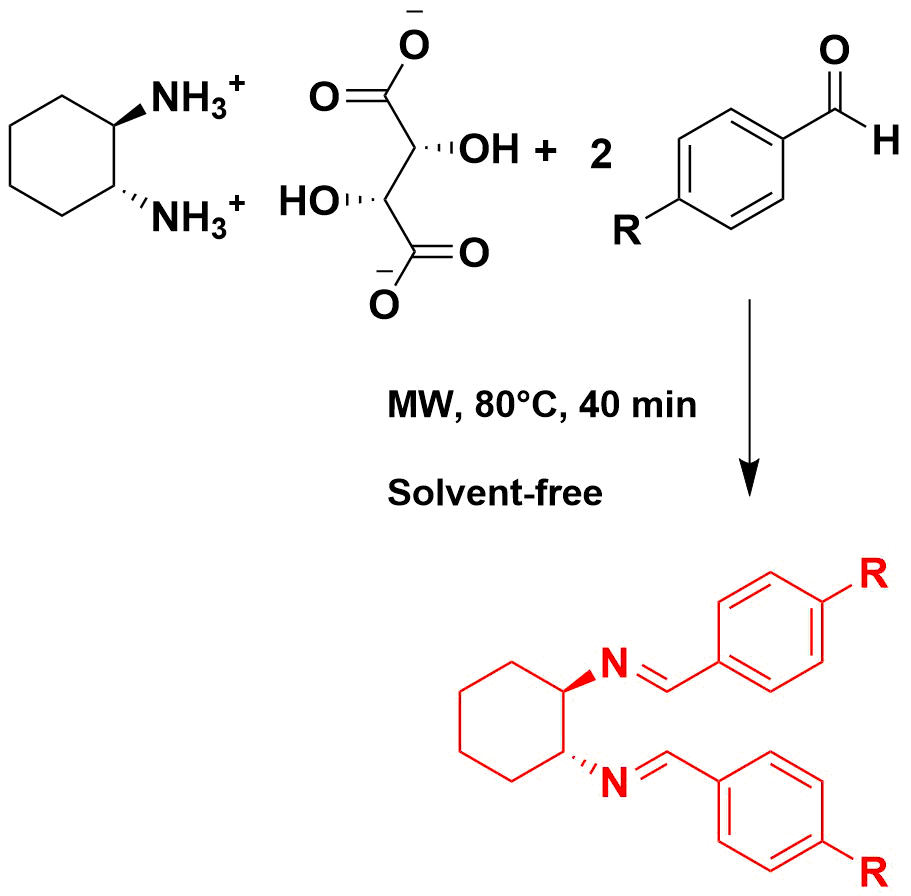
Graphycal Abstract: In this work, the synthesis of Schiff bases derived from trans-(R,R)-diaminocyclohexane by microwave irradiation (MW) is presented. The reaction yields varied between 31% and 69%, being influenced by the electronic nature of the substituents (H, Cl, Br, NO2, MeO, t-BuO, BnO, and 4-(4-Me)PhO) and the reaction temperature. The spectrophotometric properties of the products were investigated by UV-Vis spectrophotometry, revealing bathochromic and hypso-chromic effects attributable to the different substituent groups. These effects were interpreted by DFT calculations with the B3LYP functional at the 6-311G(d,p) level. The results suggest that the electronic properties of the substituents in the para position have a significant impact on the spectroscopic characteristics of the Schiff bases.

Downloads: 24
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2025 Volume 20, no.1
Pages: 62-68
Stefan Robu, Tamara Potlog, Ion Bulimestru, Ion Lungu, Olga Sadohina, Alexandrina Druta, Petru Bulmaga, Iacob Gutu
Field: Organic chemistry
Type: Research paper
Issue: 2025 Volume 20, no.1
Pages: 62-68
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2025.1217
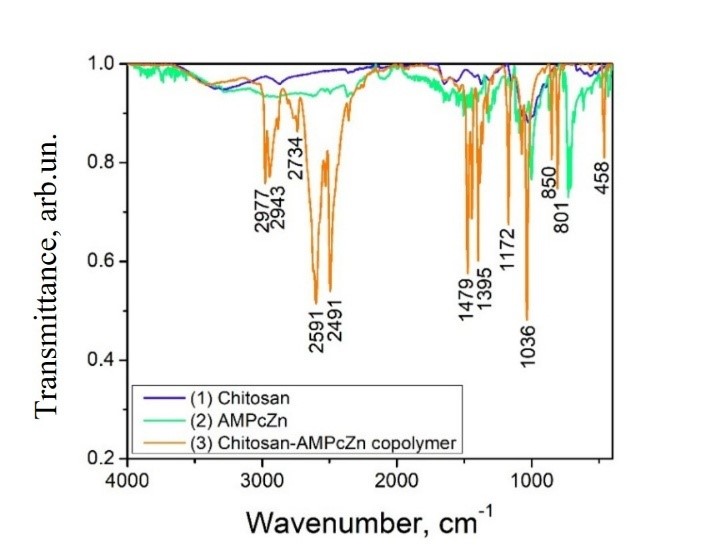
Graphycal Abstract: This study presents the synthesis of a novel polymer analogue derived from aminomethyl zinc phthalocyanine (AmPcZn) and chitosan (CH). The polymer was produced by grafting AmPcZn onto chitosan using ethyl chloroformate as a coupling agent. The resulting CH–AmPcZn polymers, containing 10%, 20%, 30%, and 60% AmPcZn, were characterized using FTIR and UV-Vis spectroscopy. The UV-Vis analysis showed that absorbance increased with higher concentrations of AmPcZn in the CH–AmPcZn solutions.

Downloads: 72
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.2
Pages: 83-92
Mohamed Loughzail, Koffi Senam Etsè, Zaragoza Verez Guillermo, Rachid Touzani, Anna Moliterni, Mohamed Anouar Harrad, Abdessamad Tounsi
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.2
Pages: 83-92
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2024.1220
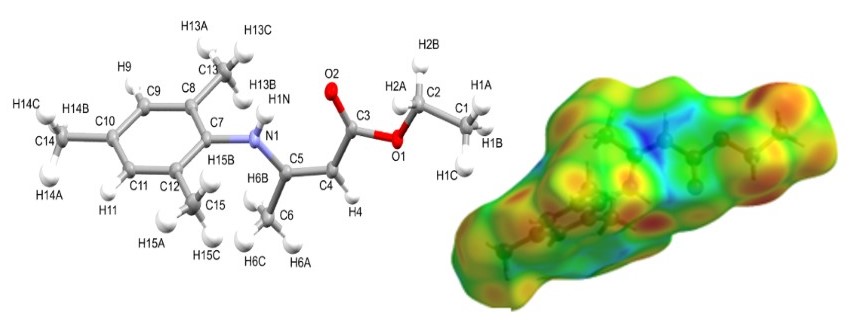
Graphycal Abstract: The compound 3-(2,4,6-timethyl-phenylamino)-but-2-enoate was obtained by the condensation reaction of ethyl acetoacetate and 2,4,6-trimethyl-phenylamine. X-ray structural analysis identified the structure of the synthesized β-enaminoester, NMR spectroscopy complemented it, and the structure stabilised by intramolecular interactions. The intermolecular contacts were further analysed by the mapping of contacts descriptors dnorm, de, di, the shape-by-shape index and surface property by electrostatic potential mapped on the Hirshfeld surface (HS). Global reactivity factors such as electronegativity, chemical hardness, potential, and softness were calculated using density functional theory. The effects of the molecular environment were accessed by analysing the electrostatic potentials surface mapped over the HS and the 3D-topology of energy frameworks. As a potential bioactive molecule, the physicochemical and ADME-Tox predictions were performed suggesting that title compound could be considered a promising drug candidate.
Downloads: 112
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.2
Pages: 74-82
Mohamed Anouar Harrad, Adnan Semane, Mohammed Badereddine, Abdessamad Tounsi
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.2
Pages: 74-82
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2024.1199
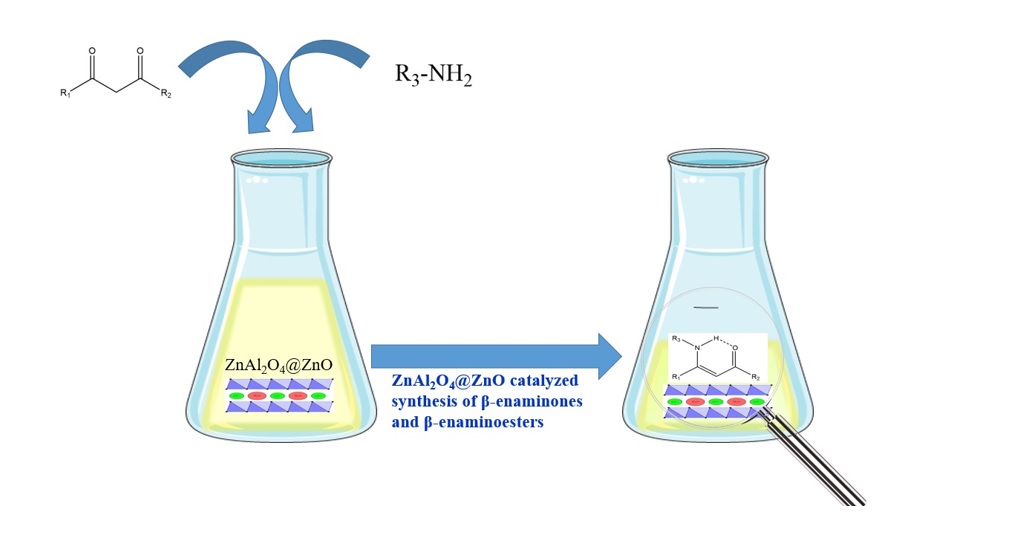
Graphycal Abstract: With ZnAl2O4@ZnO as a catalyst, an environmentally eco-friendly and highly effective method was developed for regio-and chemo-selective bis enamination of 1,3-dicarbonyl compounds and aromatic, aliphatic primary amines. A wide variety of bis-(β-enaminones) and bis-(β-enaminoesters) can be synthesized using this highly versatile method which offers good yields.
Downloads: 78
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.1
Pages: 76-83
Nuzhat Rehman, Ayaz Mahmood Dar, Raja Feroz Ahmad Haji, Sharief-ud-din Khan, Deepak Pareek, Saleem Farooq, Bashir Ahmad Dar
Field: Organic chemistry
Type: Research paper
Issue: 2024 Volume 19, no.1
Pages: 76-83
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2024.1135
Abstract (PDF)
Supplementary Material (PDF)
Graphical Abstract: This study presents an eco-friendly method for synthesizing 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) through the Biginelli reaction. A novel Heteropolyacid-Clay (HPA-Clay) catalyst, formed by immobilizing H5PV2W10O40 on Montmorillonite KSF clay, displays enhanced stability and catalytic efficiency. Operating under solvent-free, one-pot conditions, the process delivers DHPMs with high yields and shortened reaction times. Catalyzed by 2 mol% HPA-Clay, it adheres to green chemistry principles, emphasizing cost-efficiency, environmental sustainability, and recyclability. The catalyst consistently performs over multiple cycles, showcasing promise for advancing Biginelli reactions.
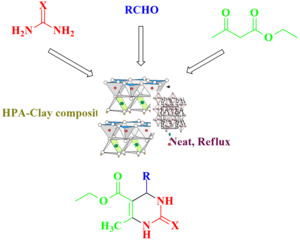
Abstract (PDF)
Supplementary Material (PDF)
Graphical Abstract: This study presents an eco-friendly method for synthesizing 3,4-dihydropyrimidin-2(1H)-ones (DHPMs) through the Biginelli reaction. A novel Heteropolyacid-Clay (HPA-Clay) catalyst, formed by immobilizing H5PV2W10O40 on Montmorillonite KSF clay, displays enhanced stability and catalytic efficiency. Operating under solvent-free, one-pot conditions, the process delivers DHPMs with high yields and shortened reaction times. Catalyzed by 2 mol% HPA-Clay, it adheres to green chemistry principles, emphasizing cost-efficiency, environmental sustainability, and recyclability. The catalyst consistently performs over multiple cycles, showcasing promise for advancing Biginelli reactions.

Downloads: 94
Author(s):
Field: Organic chemistry
Type: Review
Issue: 2024 Volume 19, no.1
Pages: 9-28
Serghei Pogrebnoi
Field: Organic chemistry
Type: Review
Issue: 2024 Volume 19, no.1
Pages: 9-28
Full Text (PDF): Download
https://doi.org/10.19261/cjm.2024.1139
Abstract (PDF)
Graphical Abstract: The review is dedicated to the synthesis of 1,3-diaryl-2-propen-2-ones derived from aromatic methyl ketones. The article highlights advancements in the synthesis of chalcones and hybrid compounds based on chalcones containing 1,2,4-triazole, tetrazole-pyrazoline, and chromenol moieties. The biological activity of the synthesized compounds is comprehensively discussed.
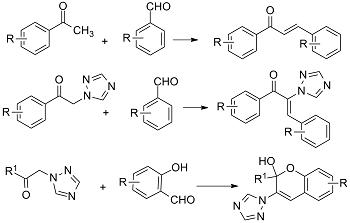
Downloads: 91
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2022 Volume 17, no.2
Pages: 101-108
Rajendra Patil, Jagdish Chavan, Shivnath Patel, Vaishali Shinde, Anil Beldar
Field: Organic chemistry
Type: Research paper
Issue: 2022 Volume 17, no.2
Pages: 101-108
Full Text (PDF): Download
Graphical Abstract: A green and efficient method for the multicomponent synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones using acetic acid supported on activated charcoal as a mild acid catalyst in ethanol under both conventional as well as microwave irradiation conditions has been developed. The catalyst system found more efficient under microwave irradiation conditions than conventional conditions with shorter reaction times and excellent yields.
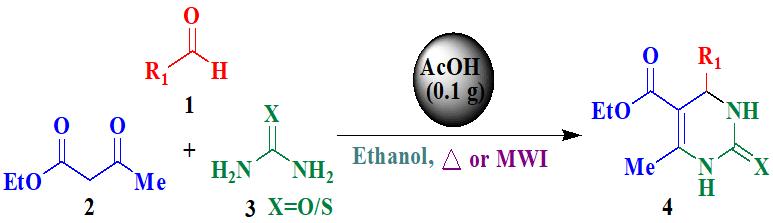
Downloads: 151
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2022 Volume 17, no.2
Pages: 94-100
Dhanraj Kamble, Anil Shankarwar, Yuvraj Sarnikar, Radhakrushna Tigote, Mubarak Shaikh, Pravin Chavan
Field: Organic chemistry
Type: Research paper
Issue: 2022 Volume 17, no.2
Pages: 94-100
Full Text (PDF): Download
Graphical Abstract: Herein, an efficient one-pot synthesis is described, of substituted benzimidazole derivatives (3a-j) from a condensation of various o-phenylenediamine (1a-j) aromatic aldehyde (2a-j) using ZnFe2O4 as a nano-catalyst under ultrasonic irradiation conditions. All forms of aldehydes with an electron releasing or electron –withdrawing substituent have a significant yield. The catalyst can easily be recovered after completion of the reaction and reused without affecting its activity. Prepared benzimidazole derivatives showed moderate to good anti-tuberculosis results.
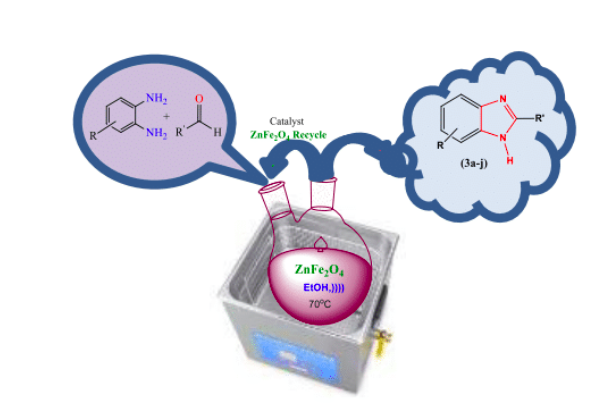

Downloads: 119
Author(s):
Field: Organic chemistry
Type: Research paper
Issue: 2022 Volume 17, no.1
Pages: 56-61
Silvadas Jesna Das, Mohan Sidharth, Chandroth Kalyad Simi
Field: Organic chemistry
Type: Research paper
Issue: 2022 Volume 17, no.1
Pages: 56-61
Full Text (PDF): Download
Abstract (PDF)
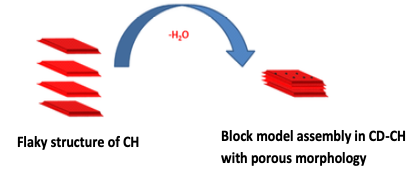
Graphical Abstract: A facile, one-pot, and solvent-free synthesis was developed to obtain a thermally stable chitosan biopolymer. The bifunctional isocyanate by interaction with chitosan formed urea and urethane bonds between chitosan chains. Subsequently, the designed chemistry facilitated the formation of carbodiimide bonds between chitosan chains via dehydration of the urea bond. The modified chitosan was proved to be superior in thermal properties and could be used as a thermally stable bio-filler. This synthetic methodology is a facile route to achieve improved thermal stability in biopolymers.
Graphical Abstract: A facile, one-pot, and solvent-free synthesis was developed to obtain a thermally stable chitosan biopolymer. The bifunctional isocyanate by interaction with chitosan formed urea and urethane bonds between chitosan chains. Subsequently, the designed chemistry facilitated the formation of carbodiimide bonds between chitosan chains via dehydration of the urea bond. The modified chitosan was proved to be superior in thermal properties and could be used as a thermally stable bio-filler. This synthetic methodology is a facile route to achieve improved thermal stability in biopolymers.
Downloads: 144
Author(s):
Field: Organic chemistry
Type: Short communication
Issue: 2022 Volume 17, no.1
Pages: 62-66
Aparna Das, Ram Naresh Yadav, Bimal Krishna Banik
Field: Organic chemistry
Type: Short communication
Issue: 2022 Volume 17, no.1
Pages: 62-66
Full Text (PDF): Download
Abstract (PDF)
Graphical Abstract: In this work, the stereospecific synthesis of optically active cis β-lactams under diverse microwave-induced conditions using diverse solvents was reported. The effects of low tanδ values of the solvents are found to be more crucial than solvents with high dipole moments and dielectric constants. The results indicated that for the synthesis of β-lactams solvents with low tanδ and high dipole moment and high dielectric constant are necessary. Best of the knowledge this is the first report that examined the importance of tanδ values of the solvents in β-lactams synthesis.
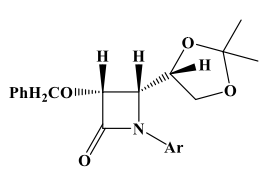
Graphical Abstract: In this work, the stereospecific synthesis of optically active cis β-lactams under diverse microwave-induced conditions using diverse solvents was reported. The effects of low tanδ values of the solvents are found to be more crucial than solvents with high dipole moments and dielectric constants. The results indicated that for the synthesis of β-lactams solvents with low tanδ and high dipole moment and high dielectric constant are necessary. Best of the knowledge this is the first report that examined the importance of tanδ values of the solvents in β-lactams synthesis.

Downloads: 117






